Introduction :
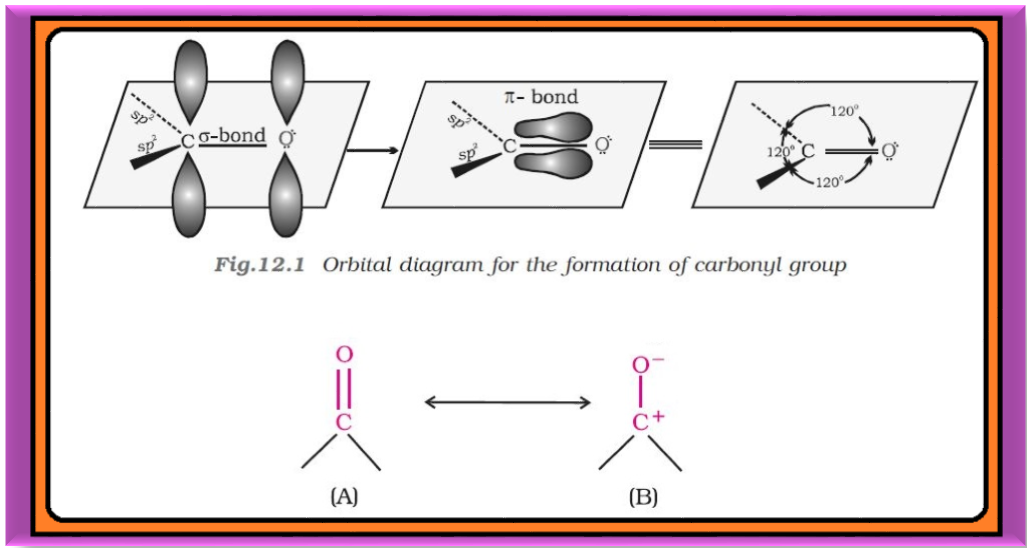
`=>` In this Unit, we will study about the organic compounds containing carbon-oxygen double bond (`color{red}(> C=O)`) called carboxyl group, which is one of the most important functional groups in organic chemistry.
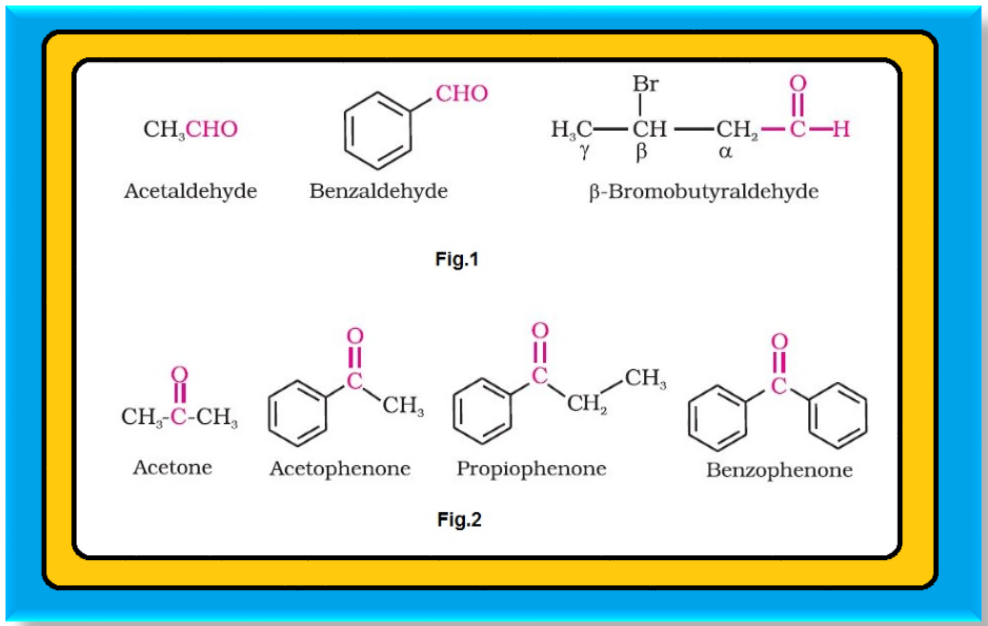
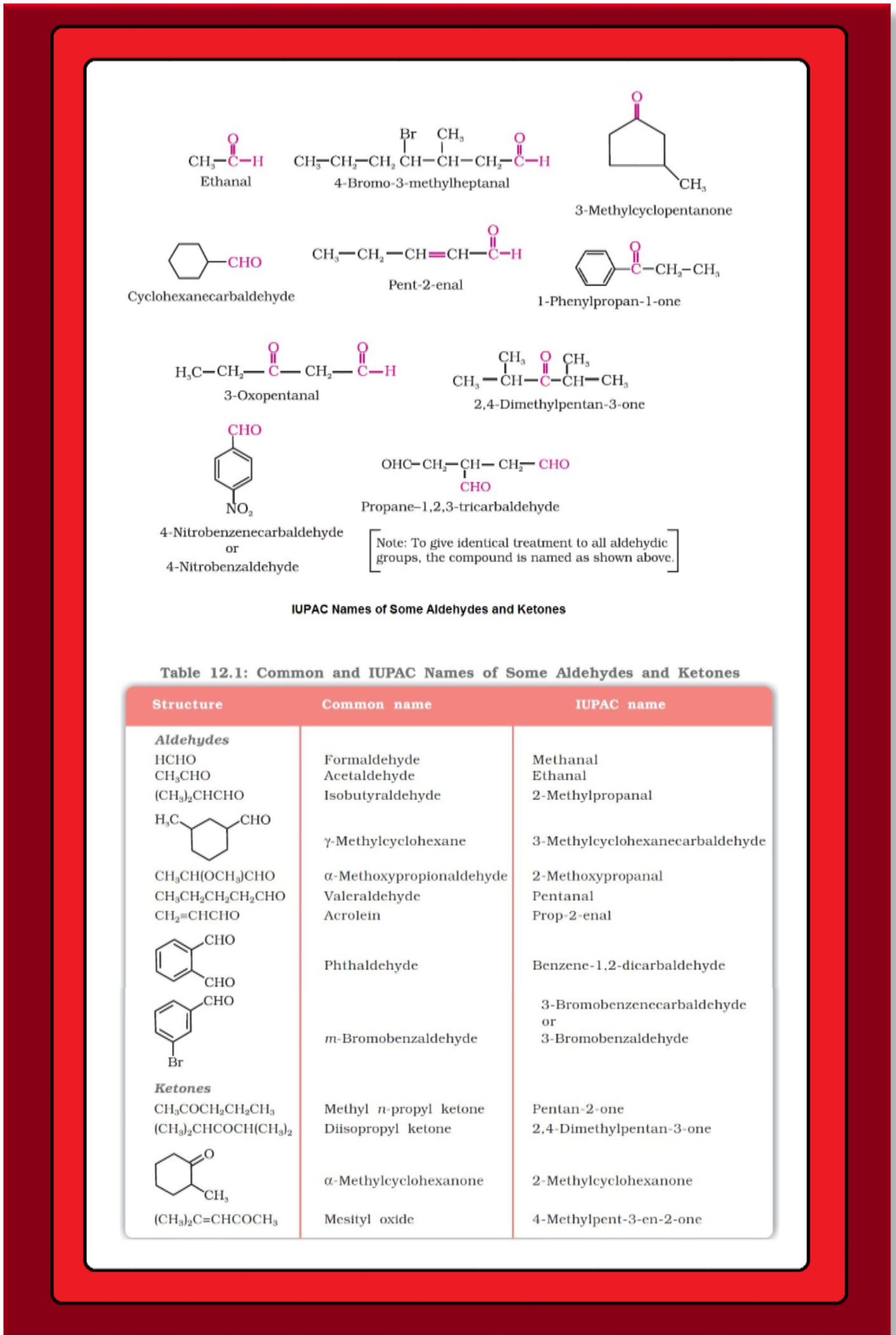
`=>` In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in the ketones, it is bonded to two carbon atoms.
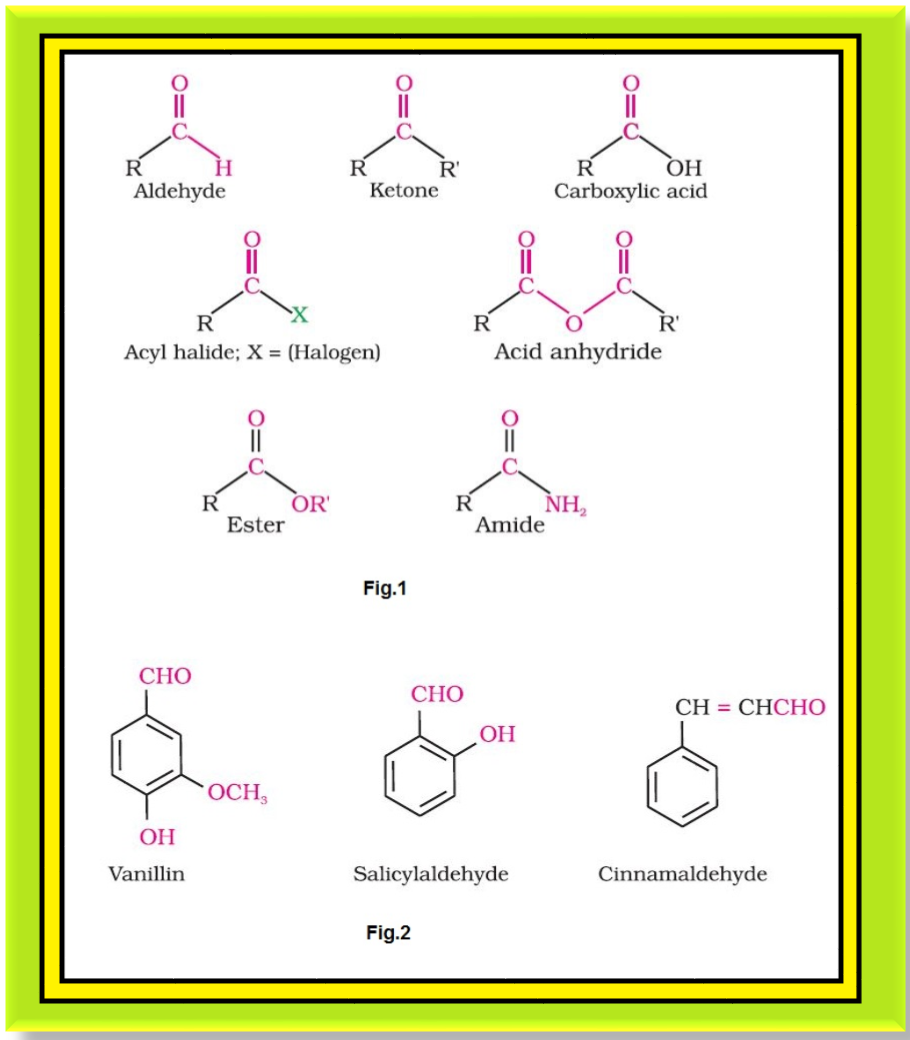
`=>` The carbonyl compounds in which carbonyl group is bonded to oxygen are known as carboxylic acids, and their derivatives (e.g. esters, anhydrides).
`=>` The carbonyl compounds in which carbonyl group is bonded to nitrogen and to halogens are called amides and acyl halides respectively. The general formulas of these classes of compounds are shown in fig.1.
`=>` Aldehydes, ketones and carboxylic acids are widespread in plants and animal kingdom.
● They play an important role in biochemical processes of life.
● They add fragrance and flavour to nature, for example, vanillin (from vanilla beans), salicylaldehyde (from meadow sweet) and cinnamaldehyde (from cinnamon) have very pleasant fragrances. See fig.2.
`=>` They are used in many food products and pharmaceuticals to add flavours.
● Some of these families are manufactured for use as solvents (i.e., acetone) and for preparing materials like adhesives, paints, resins, perfumes, plastics, fabrics, etc.
`=>` In aldehydes, the carbonyl group is bonded to a carbon and hydrogen while in the ketones, it is bonded to two carbon atoms.
`=>` The carbonyl compounds in which carbonyl group is bonded to oxygen are known as carboxylic acids, and their derivatives (e.g. esters, anhydrides).
`=>` The carbonyl compounds in which carbonyl group is bonded to nitrogen and to halogens are called amides and acyl halides respectively. The general formulas of these classes of compounds are shown in fig.1.
`=>` Aldehydes, ketones and carboxylic acids are widespread in plants and animal kingdom.
● They play an important role in biochemical processes of life.
● They add fragrance and flavour to nature, for example, vanillin (from vanilla beans), salicylaldehyde (from meadow sweet) and cinnamaldehyde (from cinnamon) have very pleasant fragrances. See fig.2.
`=>` They are used in many food products and pharmaceuticals to add flavours.
● Some of these families are manufactured for use as solvents (i.e., acetone) and for preparing materials like adhesives, paints, resins, perfumes, plastics, fabrics, etc.